Advances in WebCocon: DFT calculations
Results shown
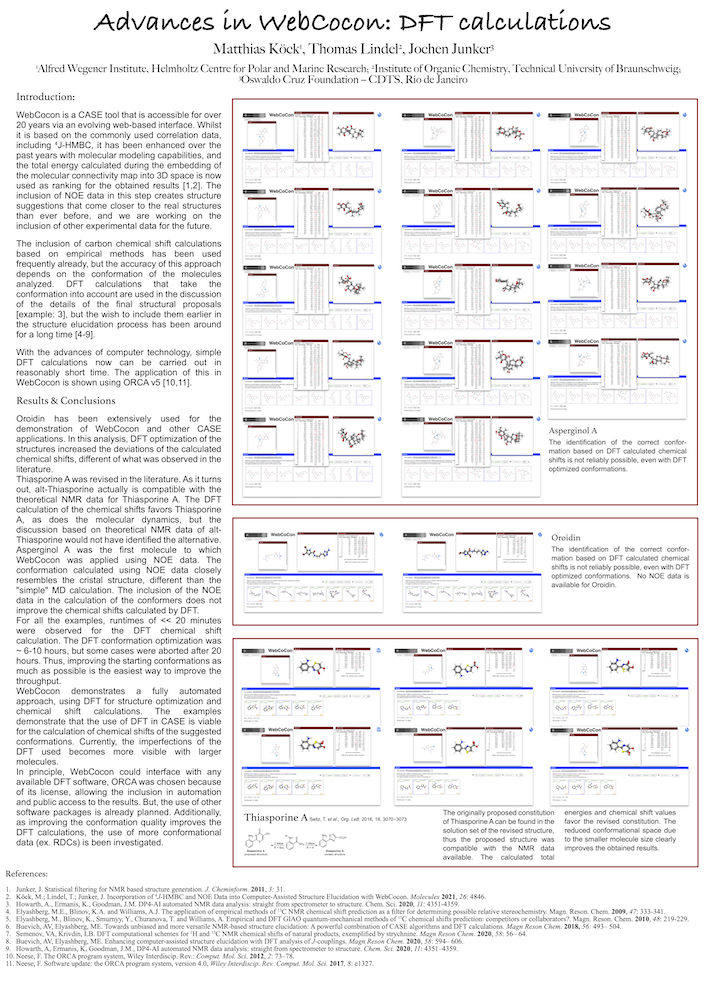
WebCocon is a CASE tool that is accessible via a webinterface. Whilst it is based on the commonly used correlation data, including 4J-HMBC, it has been enhanced over the past years with molecular modeling capabilities, and the total energy calculated during the embedding of the molecular connectivity map into 3D space is now used as ranking for the obtained results [1,2]. The inclusion of NOE data in this step creates structure suggestions that come closer to the real structures than ever before, and we are working on the inclusion of other experimental data for the future.
The inclusion of carbon chemical shift calculations based on empirical methods has been used frequently already, but the accuracy of this approach depends on the conformation of the molecules analyzed. DFT calculations that take the conformation into account are used in the discussion of the details of the final structural proposals [example: 3], but the wish to include them earlier in the structure elucidation process has been around for a long time [4-8].
With the advances of computer technology, simple DFT calculations now can be carried out in reasonably short time. The application of this in WebCocon is shown using ORCA v5 [9,10], together with a discussion of the results obtained.
Literature cited
The following literature references are used for this poster:
- Junker, J. Statistical filtering for NMR based structure generation. J. Cheminform. 2011, 3: 31.
- Köck, M.; Lindel, T.; Junker, J. Incorporation of 4J-HMBC and NOE Data into Computer-Assisted Structure Elucidation with WebCocon. Molecules 2021, 26: 4846.
- Howarth, A., Ermanis, K., Goodman, J.M. DP4-AI automated NMR data analysis: straight from spectrometer to structure. Chem. Sci. 2020, 11: 4351-4359.
- Elyashberg, M.E., Blinov, K.A. and Williams, A.J. The application of empirical methods of 13C NMR chemical shift prediction as a filter for determining possible relative stereochemistry. Magn. Reson. Chem. 2009, 47: 333-341.
- Elyashberg, M., Blinov, K., Smurnyy, Y., Churanova, T. and Williams, A. Empirical and DFT GIAO quantum-mechanical methods of 13C chemical shifts prediction: competitors or collaborators?. Magn. Reson. Chem. 2010, 48: 219-229.
- Buevich, AV, Elyashberg, ME. Towards unbiased and more versatile NMR-based structure elucidation: A powerful combination of CASE algorithms and DFT calculations. Magn Reson Chem. 2018, 56: 493– 504.
- Semenov, VA, Krivdin, LB. DFT computational schemes for 1H and 13C NMR chemical shifts of natural products, exemplified by strychnine. Magn Reson Chem. 2020, 58: 56– 64.
- Buevich, AV, Elyashberg, ME. Enhancing computer-assisted structure elucidation with DFT analysis of J-couplings. Magn Reson Chem. 2020, 58: 594– 606.
- Neese, F. The ORCA program system, Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2012, 2: 73–78.
- Neese, F. Software update: the ORCA program system, version 4.0, Wiley Interdiscip. Rev.:Comput. Mol. Sci. 2017, 8: e1327.
The Poster
 English
English
 Deutsch
Deutsch
 Português
Português