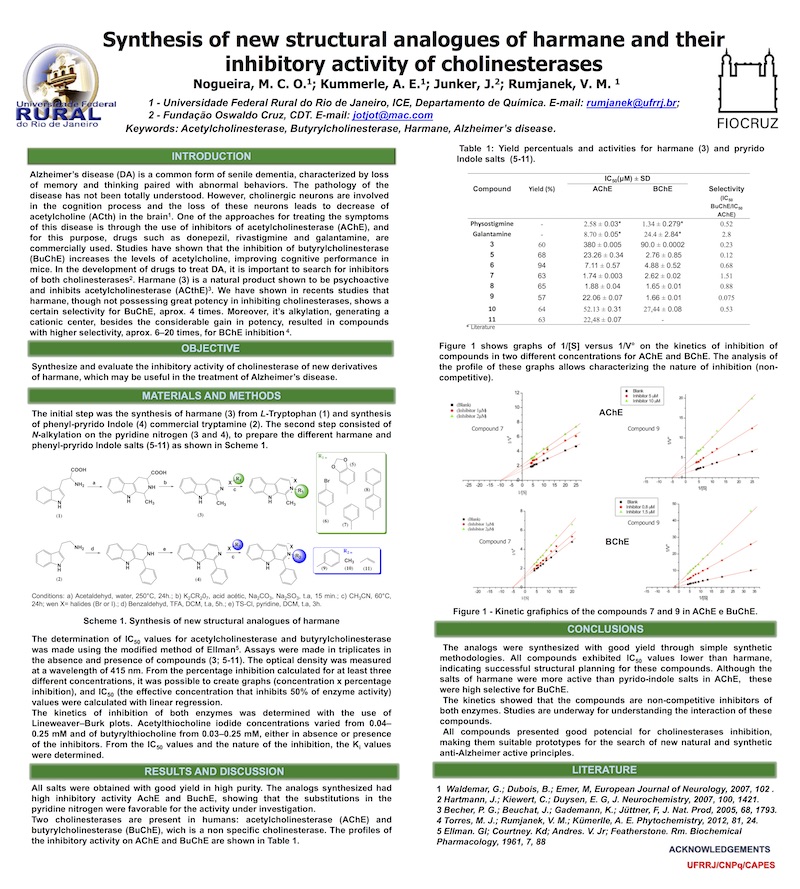
Synthesis of new structural analogues of harmane and their inhibitory activity of cholinesterases.
Results shown
Alzheimer’s disease (DA) is a common form of senile dementia, characterized by loss of memory and thinking paired with abnormal behaviors. The pathology of the disease has not been totally understood. However, cholinergic neurons are involved in the cognition process and the loss of these neurons leads to decrease of acetylcholine (ACth) in the brain1. One of the approaches for treating the symptoms of this disease is through the use of inhibitors of acetylcholinesterase (AChE), and for this purpose, drugs such as donepezil, rivastigmine and galantamine, are commercially used. Studies have shown that the inhibition of butyrylcholinesterase (BuChE) increases the levels of acetylcholine, improving cognitive performance in mice. In the development of drugs to treat DA, it is important to search for inhibitors of both cholinesterases2. Harmane (3) is a natural product shown to be psychoactive and inhibits acetylcholinesterase (ACthE)3. We have shown in recents studies that harmane, though not possessing great potency in inhibiting cholinesterases, shows a certain selectivity for BuChE, aprox. 4 times. Moreover, it’s alkylation, generating a cationic center, besides the considerable gain in potency, resulted in compounds with higher selectivity, aprox. 6–20 times, for BChE inhibition 4.
Literature cited
The following literature references are used for this poster:
- Waldemar, G.; Dubois, B.; Emer, M, European Journal of Neurology, 2007, 102 .
- Hartmann, J.; Kiewert, C.; Duysen, E. G, J. Neurochemistry, 2007, 100, 1421.
- Becher, P. G.; Beuchat, J.; Gademann, K.; Jüttner, F, J. Nat. Prod, 2005, 68, 1793.
- Torres, M. J.; Rumjanek, V. M.; Kümerlle, A. E. Phytochemistry, 2012, 81, 24.
- Ellman. Gl; Courtney. Kd; Andres. V. Jr; Featherstone. Rm. Biochemical Pharmacology, 1961, 7, 88
The Poster
 English
English
 Deutsch
Deutsch
 Português
Português